SomnusNooze
HAPPY 2023! If you’ve missed any of the latest news we’ve shared through our social media this month, don’t worry! Get up to date with the January edition of “In Case You Missed It!”
- Thank you for helping us reach our 2022 end-of-year fundraising goal!
- KemPharm is currently recruiting participants for a research study for an investigational medication for treating excessive daytime sleepiness.
- RaiseUp Study seeking participants with narcolepsy with cataplexy (NT1) for study to evaluate investigational drug treatment.
- NLS Pharmaceutics announced study results of Phase 2 clinical trials evaluating Quilience in the treatment of narcolepsy.
- Meet Dr. Gert Jan Lammers, Dr. Jason Ong, and Dr. David Rye – Members of HF’s Medical and Scientific Advisory Boards.
Don’t worry if you’ve missed anything. We’ve got you covered!
Thank you for helping us reach our end-of-year fundraising goal!
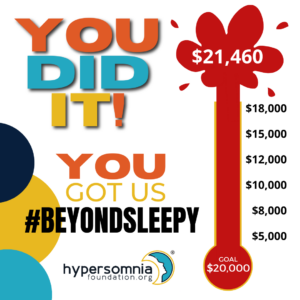 Thank you for helping us reach our end-of-year fundraising goal!
Thank you for helping us reach our end-of-year fundraising goal!
YOU DID IT! Your generosity raised $21,460! YOU got us #BeyondSleepy and WE THANK YOU!
MANY THANKS to the Rye family – Dr. David and Catherine Rye and Tony and Bertha Rye – for their generous $10,000 matching gift!
THANKS TO YOU, we entered 2023 with the support needed to continue our mission to engage, inform, and champion our global community to improve the lives of people with idiopathic hypersomnia and related sleep disorders. We are grateful for your support!
KemPharm is currently recruiting participants for a research study for an investigational medication for treating excessive daytime sleepiness

KemPharm has initiated the KP1077.D01 study to evaluate the safety and efficacy of SDX, an investigational medication for treating excessive daytime sleepiness (EDS) in people with idiopathic hypersomnia age 18 years or older.
MORE INFORMATION:
SDX has a unique, slow-release profile that could potentially provide stable control of sleepiness throughout the day, with low abuse potential. This study will evaluate the effect of SDX on symptoms and severity of IH, including the effect on brain fog, if any.
This clinical study consists of the following:
- Initial screening period during which trial participants will need to wash out all current medications.
- A five-week dose optimization phase in which all eligible study participants will receive SDX.
- A two-week double-blind randomized withdrawal phase in which study participants and physicians will not be aware of who is receiving SDX or placebo (a tablet that does not contain study drug). Participants will be further assigned into two evenly divided groups. One group will receive a single daily dose just before bedtime, and the other group will receive half the daily dose shortly after awakening and half the daily dose prior to bedtime to assess the most effective dosing regimen.
- Patients will be reimbursed for their participation, including coverage of travel costs to the clinical sites for all their clinic visits that are part of the clinical trial. Participants can talk to the staff of their clinical site to facilitate travel arrangements and reimbursements.
Details about the study and enrollment may be found on clinicaltrials.gov (NCT05668754). If interested, please contact KemPharm’s Medical Affairs team by e-mail: .
RaiseUp Study seeking participants with narcolepsy with cataplexy (NT1) for study to evaluate investigational drug treatment
 If you have narcolepsy with cataplexy (also known as narcolepsy type 1), you may be eligible to participate in research. The purpose of the RaiseUp Study is to evaluate the safety and effectiveness of an investigational study drug in the treatment of adults who have narcolepsy type 1 (NT1). This study may help researchers understand how the body processes the investigational study drug and how it may affect excessive daytime sleepiness (EDS).
If you have narcolepsy with cataplexy (also known as narcolepsy type 1), you may be eligible to participate in research. The purpose of the RaiseUp Study is to evaluate the safety and effectiveness of an investigational study drug in the treatment of adults who have narcolepsy type 1 (NT1). This study may help researchers understand how the body processes the investigational study drug and how it may affect excessive daytime sleepiness (EDS).
Who Can Participate? Eligible participants must:
- Be 18 to 70 years of age.
- Have received an NT1 diagnosis within the past 10 years or are experiencing NT1 symptoms (characterized by cataplexy, or the sudden loss of muscle control and muscle weakness due to strong emotions or actions, like anger or laughter, while remaining conscious).
- Be willing to stop any current NT1 treatment.
For more information about the RaiseUp Study or to take the prescreening questionnaire and see if you may qualify, talk to your doctor and visit our “Currently Recruiting Studies” webpage.
NLS Pharmaceutics announced study results of Phase 2 clinical trials evaluating Quilience in the treatment of narcolepsy
 NLS Pharmaceutics has announced new in vitro study results demonstrating the agonist effect of mazindol ER at the Orexin-2 Receptor. Phase 2 clinical trials evaluating Quilience (mazindol ER) in the treatment of narcolepsy recently concluded, meeting the primary endpoint with high statistical significance.
NLS Pharmaceutics has announced new in vitro study results demonstrating the agonist effect of mazindol ER at the Orexin-2 Receptor. Phase 2 clinical trials evaluating Quilience (mazindol ER) in the treatment of narcolepsy recently concluded, meeting the primary endpoint with high statistical significance.
Orexin, also known as hypocretin, is a neuropeptide that regulates arousal, wakefulness, and appetite. Orexin plays a critical role in regulating the sleep-wake cycle and the most common form of narcolepsy, type 1 (NT1), in which the individual experiences brief losses of muscle tone, known as or cataplexy, is caused by a lack of orexin in the brain due to destruction of the cells that produce it. Orexin deficit is believed to be the root cause of narcolepsy.
“This result is very encouraging for understanding the mechanism of action of mazindol ER in the treatment of narcolepsy,” said Eric Konofal, MD, PhD, Chief Scientific Officer of NLS Pharmaceutics. “This is a significant add-on therapeutic option in narcolepsy type 1 that includes excessive daytime sleepiness and the sudden loss of muscle tone, or cataplexy, when a person is awake.”
The Phase 3 program for Quilience (mazindol ER) is currently on track to commence in mid- 2023.
Read the complete announcement using this LINK.
Meet Dr. Gert Jan Lammers, Dr. Jason Ong, and Dr. David Rye—members of HF’s Medical and Scientific Advisory Boards
Meet Dr. Gert Jan Lammers — member of the Hypersomnia Foundation’s Medical Advisory Board
 Gert Jan Lammers, MD, PhD, trained as a neurologist and clinical neurophysiologist at Leiden University Medical Center (LUMC) then earned a PhD at Leiden University, with a thesis titled “Narcolepsy.” He is currently a professor of neurology at Leiden University, and the primary focus of his research is on narcolepsy and related disorders of hypersomnolence.
Gert Jan Lammers, MD, PhD, trained as a neurologist and clinical neurophysiologist at Leiden University Medical Center (LUMC) then earned a PhD at Leiden University, with a thesis titled “Narcolepsy.” He is currently a professor of neurology at Leiden University, and the primary focus of his research is on narcolepsy and related disorders of hypersomnolence.
Dr. Lammers is the medical director of the third line Sleep-Wake Centres of SEIN (Heemstede, Zwolle and Groningen), and also affiliated with the department of Neurology at LUMC. He serves as the current chair of the Dutch Society for Sleep Medicine and is co-founder of the European Narcolepsy Network, serving as president from 2007-2014.
Watch Dr. Lammers’ video presentation, “Diagnosing Hypersomnia Differently: A European Proposal,” shared during a 2021 Hypersomnia Foundation virtual webinar, where he explains the diagnostic criteria some European researchers are using for both narcolepsy and IH in patient care and research, how it differs from the standards used in the U.S., and what this means for patients. Watch his video using this LINK.
To learn more about Dr. Lammers, visit our “Medical Advisory Board” web page.
Thank you, Dr. Lammers, for your support of the Hypersomnia Foundation and your continued focus on researching narcolepsy and related disorders of hypersomnolence.
Meet Dr. Jason Ong — member of the Hypersomnia Foundation’s Medical Advisory Board
 Jason Ong, PhD, DBSM, is the Director of Behavioral Sleep Medicine at Nox Health and an Adjunct Associate Professor of Neurology at Northwestern University Feinberg School of Medicine. He received his PhD in clinical psychology from Virginia Commonwealth University and completed a fellowship in Behavioral Sleep Medicine at Stanford University Medical Center.
Jason Ong, PhD, DBSM, is the Director of Behavioral Sleep Medicine at Nox Health and an Adjunct Associate Professor of Neurology at Northwestern University Feinberg School of Medicine. He received his PhD in clinical psychology from Virginia Commonwealth University and completed a fellowship in Behavioral Sleep Medicine at Stanford University Medical Center.
His research interest involves demonstrating the effectiveness and value of behavioral treatments for sleep disorders, including cognitive-behavioral therapy and mindfulness meditation. Related to hypersomnias, Dr. Ong is involved in studies examining the psychosocial impact of hypersomnias and behavioral interventions to improve quality of life in people with hypersomnias.
Use this LINK to read Dr. Ong’s article, “What Is Sleep Hygiene Anyway?” and learn about sleep hygiene and what it means for people who have hypersomnias.
Then read an interesting interview with Dr. Ong where he discusses sleep and how a behavioral approach can improve long-term outcomes. The link to Dr. Ong’s interview with Nox Health is HERE.
To learn more about Dr. Ong, visit our “Medical Advisory Board” web page.
Thank you, Dr. Ong, for your support of the Hypersomnia Foundation and your continued focus on researching behavioral treatments for sleep disorders.
Meet Dr. David Rye — member of the Hypersomnia Foundation’s Scientific Advisory Board
 David Rye, MD, PhD, is a professor of neurology at Emory University’s School of Medicine and the director of research for Emory Healthcare’s Program in Sleep Medicine. Dr. Rye is an internationally recognized expert in narcolepsy and related disorders of excessive daytime sleepiness and movement disorders in sleep, particularly Restless Legs Syndrome (RLS).
David Rye, MD, PhD, is a professor of neurology at Emory University’s School of Medicine and the director of research for Emory Healthcare’s Program in Sleep Medicine. Dr. Rye is an internationally recognized expert in narcolepsy and related disorders of excessive daytime sleepiness and movement disorders in sleep, particularly Restless Legs Syndrome (RLS).
Dr. Rye was part of the international team that identified the first gene variant associated with RLS, a neurological condition characterized by periodic leg movements in sleep and the irresistible urge to move the legs. He also led a team that linked RLS to high blood pressure and related vascular risks. In June of 2019, Dr. Rye received the Hypersomnia Foundation’s first Impact Award, for his pivotal work researching and treating idiopathic hypersomnia, and for his support of HF’s efforts to provide education on IH and related disorders to patients, health providers and, through PBS’s “Your Fantastic Mind” series, to the general public.
His current research focuses on RLS and hypersomnia. Dr. Rye is committed to advancing our understanding of the genetic and pharmacological underpinnings for sleep and its disorders with an eye on recognizing and improving treatment choices for patients.
Use this LINK to read “A Life Consumed by Sleep” in the Emory Health Sciences Research Blog “Lab Land,” which shares the experience of a patient from Iceland who travelled to Atlanta specifically to see Dr. Rye in hope of finally getting answers and help for his excessive sleepiness.
To view multiple presentations by Dr. Rye, use this LINK to our “Videos & Podcasts” webpage.
For more information about Dr. Rye, visit our “Scientific Advisory Board” webpage.
We are extremely grateful for Dr. Rye and his dedication to the Hypersomnia Foundation and the sleep disorder community.
