SomnusNooze
New research on genetically altered mice helps to untangle the complex interactions of GABA with brain systems necessary for sustaining wakefulness.
Histamine
Nerve cells (also known as neurons) in a specific part of the brain, called the tuberomammilary nucleus or TMN, produce histamine. Histamine is a neurotransmitter—a chemical messenger that nerve cells use to communicate with each other. Histamine is an excitatory neurotransmitter and is one of the monoamine family of neurotransmitters (along with dopamine and noradrenaline, for example) responsible for keeping us awake. In addition to making people, or animals for that matter, more alert, histamine also promotes eating (or feeding), motivation, and the ability to think clearly (cognition). Although histamine is only produced in the TMN, histaminergic or histamine-producing neurons have long branches, or axons, that branch extensively throughout the brain and spinal cord, so these histaminergic neurons can effectively “talk to” widespread areas of the nervous system and coordinate actions between them. Unlike cells that release neurotransmitters into the space between two nerve cells (an area known as the synapse or synaptic cleft), TMN axons release histamine via volume transmission, meaning that they basically flood the area around them with histamine. Instead of binding with receptors at the synapse, this histamine binds to receptors outside the synapse (known as extrasynaptic receptors). Very little transmission of histamine takes place at synapses.
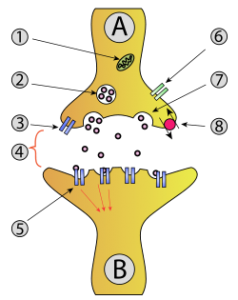 Synaptic transmission: A and B are both nerve cells or neurons. A comes before the synapse (4) and is therefore termed presynaptic and B is postsynaptic, meaning that the information is being sent from A, across the space or synapse, to B. The number 2 highlights the packets or vesicles that hold the neurotransmitter or chemical in the presynaptic neuron. 7 indicates the vesicle moving to the surface of the presynaptic neuron, where it “pops” and releases its contents into the synapse (4). 5 shows the receptor on the postsynaptic neuron in which the chemical docks and delivers its signal.
Synaptic transmission: A and B are both nerve cells or neurons. A comes before the synapse (4) and is therefore termed presynaptic and B is postsynaptic, meaning that the information is being sent from A, across the space or synapse, to B. The number 2 highlights the packets or vesicles that hold the neurotransmitter or chemical in the presynaptic neuron. 7 indicates the vesicle moving to the surface of the presynaptic neuron, where it “pops” and releases its contents into the synapse (4). 5 shows the receptor on the postsynaptic neuron in which the chemical docks and delivers its signal.
GABA
TMN neurons also contain GABA (γ-aminobutyric acid), but we don’t really know what the role of GABA is in these cells or whether these cells actually even release GABA. In humans, GABA is also directly responsible for regulating muscle tone. Scientists at Emory University have identified a substance, as yet unnamed, that is often present in the cerebrospinal fluid of people with idiopathic hypersomnia and even a majority of people with a certain type of narcolepsy (viz., Type 2 narcolepsy) and Kleine-Levin syndrome. This substance appears to increase the power, or potentiate the effects, of GABA in the brain. The Emory scientists have introduced the term “GABA-related hypersomnias” in referring to these disorders.
Vesicular GABA Transporter Genes
A gene in mice, called vgat, provides the blueprint, or codes, to build a protein called vesicular GABA transporter (or vGAT). (In humans, the gene responsible for coding of vGAT is called SLC32A1.) vGAT is present in high concentrations in the nerve endings of GABAergic neurons in the brain and spinal cord and is necessary for GABA to be taken up into synaptic vesicles in order for it to then be released from nerve endings. (See the illustration below this story for more information on synaptic vesicles).
The New Research
A group of scientists in England wondered what would happen if they manipulated the vgat gene in mice so that the gene was not fully expressed (meaning that the instructions to cells to produce vGAT were decreased from the normal level) or if the gene was completely missing. They performed a series of experiments to answer their questions. In one group of mice, the scientists performed a “gene knockdown” of the vgat gene, not simply in the whole animal (where it was technically not feasible and might have been lethal), but, rather, selectively in the TMN. This procedure resulted in the vgat gene not being fully expressed in neurons residing in the TMN.
In a second group of animals, the scientists created a “knockout” mouse by completely inactivating the vgat gene, again, with the intent of selectively inactivating the gene in the TMN.
The scientists then compared these two groups of knockdown and knockout mice (experimental groups) with a group of mice that had not had any procedures performed on them (the control group). They put each mouse in a test chamber to measure the mouse’s activity level. They calculated how long the mouse moved around and how much distance it covered. The experimental groups of mice had significantly more movement than the control mice. They also moved faster. The scientists note in their paper that the mice had “stable and high activity” throughout the test. In a second set of experiments using the same groups of mice, the scientists limited the amount of sleep that the mice had (sleep deprivation) and then put the mice back into their cages and allowed them to sleep. The experimental mice stayed awake much longer than did the control mice, even after they were allowed to sleep. They also had significantly less non-rapid eye movement or non-REM sleep and failed to catch up on their “lost” sleep. The scientists described the experimental mice as “hyperactive” and “sleepless.” The results strongly suggest that GABA released from histaminergic neurons governs the amount of sleep–and by inference–the amount and quality of wake. The scientists suggest that these components work together, in balance, to regulate sleep/wakefulness.
The New Research in Context – Relevance to Clinical Hypersomnia
Although the results of this study do not immediately suggest any new treatment options for people with GABA-related sleep disorders, they highlight the growing interest and efforts of basic neuroscientists in untangling the complex interactions of GABA with brain systems necessary for sustaining wakefulness. The same group of researchers, for example, has previously recently shown that GABA-A receptors on TMN neurons, although important for sustaining wakefulness, do not appear necessary for inducing anesthesia—and, by inference, sleep (AY Zecheria et al. J Neurosci 2012;32(38):13062-75. GABAergic inhibition of histaminergic neurons regulates active waking but not the sleep-wake switch or propofol-induced loss of consciousness). Taken together, these findings support the continued development of agents such as pitolisant, a histamine H3 inverse agonist designed to increase histamine release, in the treatment of both primary and secondary hypersomnias (see, for example, S Leu-Semenescu et al. Sleep Med 2014;15(6):681-7.) in whom cerebrospinal fluid histamine levels otherwise appear normal (Y Dauvilliers et al. Sleep 2012;35(10):1359-66. Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions).
Key Highlights from this Paper
• Histaminergic axons co-release GABA into the neocortex and striatum
• The released GABA produces slow tonic inhibition
• Reducing vgat expression in histaminergic neurons increases wakefulness
• Histamine-GABA axons will coordinate tonic inhibition over large cortical areas
Thank you to Sarah Neena-Koch for the allowing us to use this figure from her website, MyBrainNotes.com. Lots of interesting material here if you would like to learn more about the brain!
Wakefulness is governed by GABA and histamine cotransmission. Neuron 2015:87(1):164–178. You can download or read the full paper at https://www.cell.com/neuron/pdf/S0896-6273(15)00516-4.pdf
