SomnusNooze e-newsletter
News, stories and updates from the world of sleep
News, stories and updates from the world of sleep

Narcolepsy vs Idiopathic Hypersomnia: What's the Difference? My 9-year-old son recently was diagnosed with IH but can't exclude narcolepsy. We got a second opinion and the doctor agreed. I don't understand why they don't have a definitive answer. The... read >
Question: Is there an overlap between idiopathic hypersomnia and type 2 narcolepsy? Answer: The short answer to this question is yes. The only way to definitively distinguish idiopathic hypersomnia (IH) from type 2 narcolepsy (T2N) is the number of... read >

Rather than writing our own article for this week’s edition of SomnusNooze, we are bringing you information from Dr. David Cunnington in Melbourne, Australia. Dr. Cunnington has agreed to share with us a recent post from his website (sleephub.com.au)... read >
During the presentation by David Rye, MD, PhD, titled “What are the latest developments in research on idiopathic hypersomnia?” at the Beyond Sleepy in the Mile-High City Hypersomnia Conference, he pointed out that, while on the one hand without a... read >
Very recently, the Hypersomnia Foundation became aware of an opportunity to help shape the future of sleep research. The National Institutes of Health, the primary source of funding for medical research in the United States, has issued a Request... read >
Background According to the Centers for Disease Control and Prevention (CDC), more than 2 million people in the United States suffer a traumatic brain injury (TBI) every year. Most people with a TBI will also experience a sleep-wake disturbance... read >
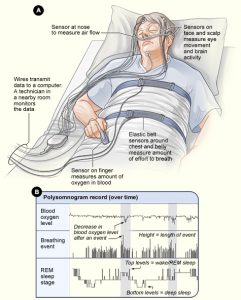
Background The primary feature of the two main central disorders of hypersomnolence—narcolepsy and idiopathic hypersomnia (IH)—is excessive daytime sleepiness, defined in the International Classification of Sleep Disorders, third edition,... read >

Background In this 2013 study published in PLoS One, the researchers Miyagawa et al hypothesized that L-carnitine supplementation might help improve narcolepsy symptoms by increasing β-fatty-acid oxidation. Fatty acids are a major energy source for... read >
On November 20, 2015, the European Medicine Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended that Bioprojet Pharma's drug pitolisant (Wakix) be approved for the treatment of narcolepsy with or without cataplexy. In... read >
Background Sodium oxybate (SXB) is a drug sold as Xyrem in the United States by Jazz Pharmaceuticals, in Europe by UCB Pharma, and in Canada by Valeant Pharmaceuticals. It is approved in the United States in adults for the treatment of excessive daytime... read >