SomnusNooze e-newsletter
News, stories and updates from the world of sleep
News, stories and updates from the world of sleep
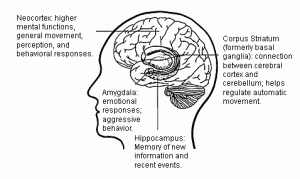
A Pilot Study of tDCS Looks Promising for the Treatment of Idiopathic Hypersomnia Background Idiopathic hypersomnia (IH) can severely impact affected individuals’ family, employment, education, and leisure activities. And because there are no... read >
Question: Is there an overlap between idiopathic hypersomnia and type 2 narcolepsy? Answer: The short answer to this question is yes. The only way to definitively distinguish idiopathic hypersomnia (IH) from type 2 narcolepsy (T2N) is the number of... read >